Two newly discovered forms of frozen salt water could help scientists solve a mystery related to… Solar System Ice-covered moons.
When subjected to higher pressures and lower temperatures than it would otherwise be found in nature On Earth, the atoms in hydrated sodium chloride — more commonly known as salt water ice — have arranged themselves into previously unrecognized structures that contain a much higher percentage of water molecules than salt.
This may explain the strange chemical signature of material on the surface of Jupiter’s moon Europa, which appears more watery than scientists expect.
“It is rare nowadays to have fundamental discoveries in science,” Earth and space scientist Baptiste Journeau says from the University of Washington.
“Salt and water are well known under Earth conditions. But then, we’re completely in the dark. And now we have these planetary bodies that probably contain compounds that are very familiar to us, but in very strange conditions. We have to bring back all the basic mineral science that people did in the ninth century. th, but at high pressure and low temperature. It’s an exciting time.”
Salt and water – also known as sodium chloride and hydrogen peroxide – are abundant in our home world. When combined, the salt molecules dissolve through the water molecules to form a solution. The presence of salt lowers the freezing point of a solution compared to unsalted water, but as the temperature continues to drop under typical Earth’s weather conditions, it will eventually freeze.
When it does, the molecules arrange themselves into a rigid lattice structure known as a hydrate. On Earth (outside the lab), this structure has only one composition: one salt molecule for every two water molecules.
On moons such as Europa and Ganymede, which orbit Jupiter, and Saturn’s moon Enceladus, scientists have also found evidence of salt and water, only the conditions found are somewhat different from those on Earth.
The surfaces of these distant worlds in the near-vacuum of space, far from the Sun, are exposed to extreme cold. Beneath their icy coats are oceans which in some cases can be more than 100 times thicker than The deepest waters on earthleading to some very extreme pressures and temperatures.
Journaux and colleagues set out to investigate the effect of salt on ice production. They squeezed a small block of salt water into a coil Diamond anvil cell Under extremely cold conditions, it generates pressures of up to 25,000 times Earth’s atmospheric pressure while lowering temperatures to -123 °C (-190 °F).
They didn’t expect what happened next.
“We were trying to measure how adding salt changes the amount of snow we can get, because salt acts as an anti-freeze,” she says. Journaux explains. “Surprisingly, when we did the pressure, what we saw was that these crystals that we didn’t expect started to grow. It was a very serendipitous discovery.”
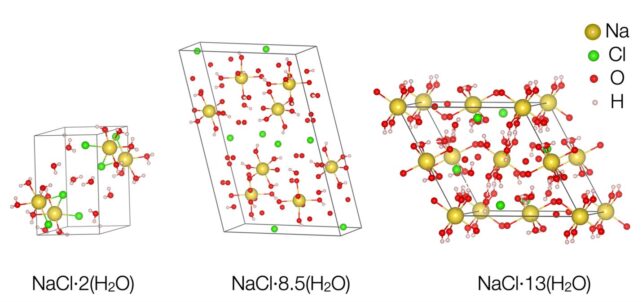
Under the conditions of their experiment, the researchers saw the emergence of two new salt and water molecule arrangements. One showed two salt molecules for every 17 water molecules. The other contains 13 water molecules for one salt molecule. Both are very different than a single salt, and they are water seen naturally on Earth – and are consistent with the water chemical signatures observed on ice moons.
“It has the structure that planetary scientists have been waiting for,” Journaux adds.
The key factor, researchers say, is pressure, which crushes molecules together and forces them to find new ways to coexist. But even when the pressure was released, one of the newly identified hydrates – containing 17 water molecules – remained stable down to temperatures of -50 degrees Celsius. This indicates that they can be found here on Earth as well, possibly under the ice of Antarctica.
Future research is needed to determine if this discovery can solve the mystery of the icy moon.
“[The hydrate’s] The infrared spectra remain to be determined in future studies,” write the researchers“But their highly hygroscopic structure could solve the long-standing mystery of the indeterminate hydrate phase on the surface of Europa and Ganymede.”
Research published in Proceedings of the National Academy of Sciences.

“Beer fan. Travel specialist. Amateur alcohol scholar. Bacon trailblazer. Music fanatic.”
